More detail about who should get this vaccine
More detail about intussusception
A history of certain gastrointestinal and bowel disorders may make this vaccine unsuitable for some infants. These include those with:
- a history of intussusception, where part of the bowel folds in on itself, like a collapsible telescope
- gastrointestinal malformation that could predispose them to intussusception.
The first dose of Rotarix is always given before 15 weeks of age, as the side effect of intussusception seems unlikely to occur if the vaccine doses start at this age. This also reduces the chance of the vaccine being wrongly blamed for cases of intussusception that sometimes occur naturally at around five months of age. There are strict rules about the age at which the rotavirus vaccine should be given to babies, to avoid the risk of intussusception.
When compared to the number of cases that happen anyway, 120 per 100,000 children, this is a very low additional risk and should be compared to the benefits of the vaccine in preventing severe rotavirus infection.
More detail about the vaccine
The vaccine contains live human rotavirus that has been weakened (attenuated), so it stimulates the immune system but does not cause disease in healthy people.
However, it should not be given to people who are clinically immunosuppressed, either due to drug treatment or underlying illness. This is because the vaccine strain could replicate too much and cause a serious infection. This includes babies whose mothers have had immunosuppressive treatment while they were pregnant or breastfeeding.
For more information see the MHRA's Drug Safety Update (April 2016) .
SCID screening
Babies should have the results of their screening for Severe Combined Immunodeficiency (SCID) checked before the rotavirus vaccine is given if they live in an area of England where screening happens. If SCID is suspected, the vaccine should not be given. However, if no SCID result is available, the vaccine should be given.
SCID screening was added to the routine newborn screening test at five days of age, in six areas across England in September 2021 – these include babies born in the regions served by screening labs in Newcastle, Manchester, Sheffield, Birmingham, Great Ormond Street Hospital and Guys and St Thomas Hospital. This is part of an evaluation that will find out if screening for SCID will work in England the same as it has in many other countries.
SCID screening is used to identify babies with a rare genetic condition that affects the immune system. This could pose a risk to them if they receive live vaccines, like the rotavirus vaccine. Babies in non-screening areas will be assigned a ‘SCID screening not offered’ result.
The rotavirus vaccine should also not be given to babies of mothers that used medication that impacts the immune system (immunosuppressive biological therapy) during their pregnancy because this could influence the infant’s immune status.
Other rotavirus vaccines available
Alongside Rotarix, another vaccine is licensed to protect against Rotavirus but is not currently used in the UK. RotaTeq is used in some countries and is delivered as a three-dose course. Where possible, infants should receive the same vaccine for each of their doses. See the RotaTeq patient information leaflet for more information.
Impact of the rotavirus vaccine
In the UK, when the rotavirus vaccine was introduced, experts predicted that the vaccine would:
- halve the number of rotavirus cases seen by GPs each year. Before a vaccine was introduced, 130,000 UK children a year visited their GP with rotavirus infection.
- cut the number of hospital admissions by two-thirds. Before introducing a vaccine, around 12,700 UK children were hospitalised with rotavirus infection a year.
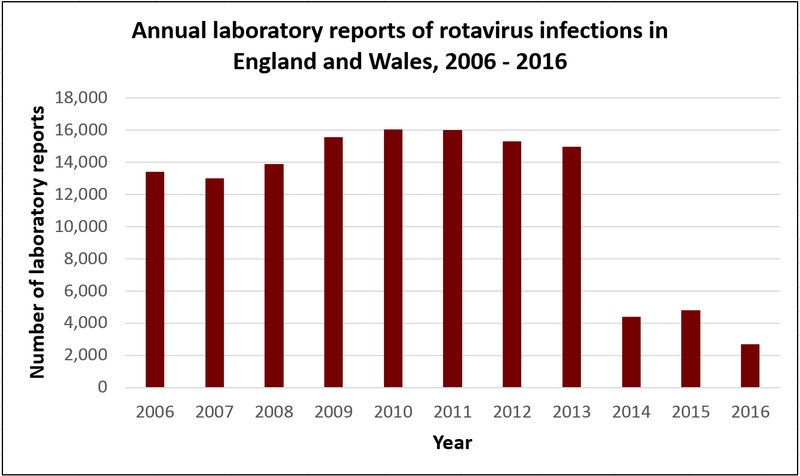
The graph below shows what has happened in the UK since the rotavirus vaccine was introduced in July 2013. In 2014, 2015 and 2016, the number of reported cases of rotavirus fell by over 70% compared to previous years. Rotavirus infections tend to peak between January and March, but in these years, there was no significant peak in cases.
For more information see the 2015 study showing the rapid decline in rotavirus infection from Public Health England and Imperial College London.
Click here for an accessible text version of this graph
Source: Public Health England Rotavirus data 2006 to 2015 and 2007 to 2016. and 2007 to 2016.
|